 Research Project Full Title: Geopolymer-Based Solutions for Coal Combustion Product Solidification and Stabilization
Research Project Full Title: Geopolymer-Based Solutions for Coal Combustion Product Solidification and Stabilization
Principal Investigator(s): Maria Juenger, Lynn Katz and Gaurav Sant (UCLA)
Researchers: Michelle Lacks, Monday Okoronkwo
Sponsor(s): Environmental Research and Education Foundation (EREF)
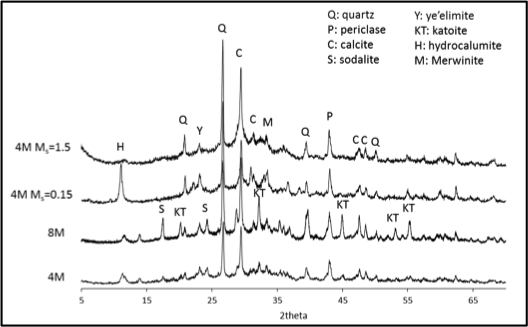
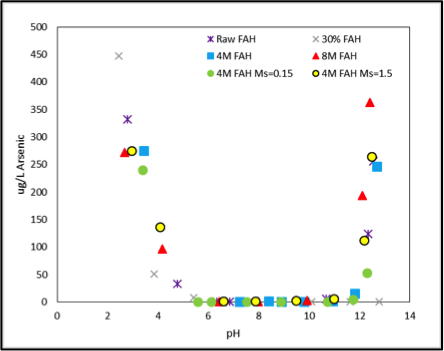
Full Abstract: Coal combustion products (CCPs) contain heavy metals that have the potential to leach into surface and ground waters when disposed improperly. Of further concern is that, in recent years, power plants have been injecting sodium carbonate compounds such as trisodium hydrogendicarbonate dihydrate (trona) into their flue gas streams to reduce SOx emissions. Trona injection has been shown to alter the characteristics of collected fly ash and increase leaching of heavy metal compounds from the ash, posing a higher environmental threat. Geopolymers that utilize CCPs as an aluminosilicate precursor are under consideration as an alternative to conventional portland cement for solidification/stabilization (S/S) of CCPs prior to disposal. These materials, made by activating aluminosiliceous powders (e.g. fly ash) with highly alkaline solutions, may improve stabilization of heavy metal wastes. The design of geopolymer mixtures for optimum S/S of CCPs is not straightforward, however. This study represents an advancement upon the traditional trial and error mixture design process by using thermodynamic phase equilibria models to predict the phases present in the solidified materials, including porosity, and experimentally validating these models. Model results were used to design mixtures for experimental testing by minimizing model predicted porosity, thereby maximizing predicted solid phase formation. Geopolymer mixtures were compared against portland cement-stabilized mixtures, and both were experimentally tested for solidification (compressive strength, phase formation, and porosity) and stabilization of heavy metals and oxyanions following EPA standard LEAF protocols at different liquid/solid (L/S) ratios and pH.
