Research Project Full Title: Adsorption Equilibrium and Kinetics at Goethite-Water and Related Interfaces
Principal Investigator(s): Frank Seibert (SRP), Lynn Katz and Kerry Kinney
Researchers: Joon Han, Sheik Mohammad Nomaan
Sponsor(s): Department of Energy, Basic Energy Sciences, DE-FG02-04ER15496
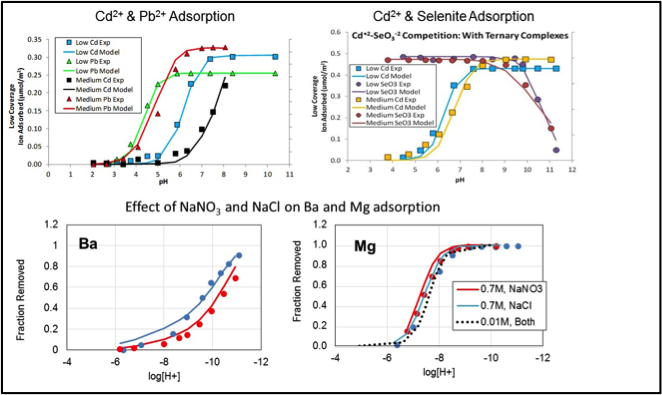
Full Abstract: The primary goal of this research study is to better understand and predict adsorption of metal ions at mineral/water surfaces. Macroscopic data in traditional batch experiments is used in conjunction with spectroscopic evidence to develop predictive models that characterize sorption in complex systems containing a wide range of background solution compositions. Our studies focus on systems involving alkaline earth metal (Mg2+, Ca2+, Sr2+, Ba2+) and heavy metal (Hg2+, Co2+, Cd2+, Cu2+, Zn2+, Pb2+) cations. The anions we selected for study included Cl–, NO3–, ClO4–, SO42-, CO32- and SeO32- and the background electrolyte cations we examined included (Na+, K+, Rb+ and Cs+) because these represent a range of ion sizes and have varying potentials for forming ion-pairs or ternary complexes with the metal ions studied. The research has led to the development of a modified titration congruency approach for estimating site densities for mineral oxides such as goethite. The CD-MUSIC version of the surface complexation modeling approach was applied to potentiometric titration data and macroscopic adsorption data for single-solute heavy metals, oxyanions, alkaline earth metals and background electrolytes over a range of pH and ionic strength. The model is capable of predicting sorption in bi-solute systems containing multiple cations, cations and oxyanions, and transition metal cations and alkaline earth metal ions. The insights gained from the macroscopic, spectroscopic and CD-MUSIC modeling developed in this study can be used to guide the implementation of less complex models which may be more applicable to field conditions. The findings of this research suggest that surface complexation models can be used as a predictive tool for fate and transport modeling of metal ions and oxyanions in fresh and saline systems typical of energy production waters and wastewaters.
