Research Project Full Title: Environmental Health and Safety Assessment of Hybrid Nanomaterials
Principal Investigator: Dr. Navid B. Saleh
Researchers: Dr. Dipesh Das, Indu V. Sabaraya (Ph.D. Candidate), Erica Mason (undergraduate student), and Sneha Jain (undergraduate student)
Sponsor(s): National Science Foundation (NSF)
Full Abstract: Extracting multifunctional benefits by combining multiple nano-scale materials has driven materials science to develop nano-heterostructures, which are known as nanohybrids (NHs). These materials in an ever-expanding space for conjugation are being researched for applications in the energy sector and in biomedical devices and processes. Among these NHs, carbon nanotubes combined with metal oxides are one of the most studied materials that provide unique advantages as electrocatalyst supports, and are currently being commercialized as embedded electrodes for fuels cells. NHs are not only a new class of complex materials but also brings in novel physicochemical properties that most likely cannot be captured by the sum of the behavior of their components materials. Thus, understanding the environmental health and safety (EHS) of this new class of composite NHs is imperative.
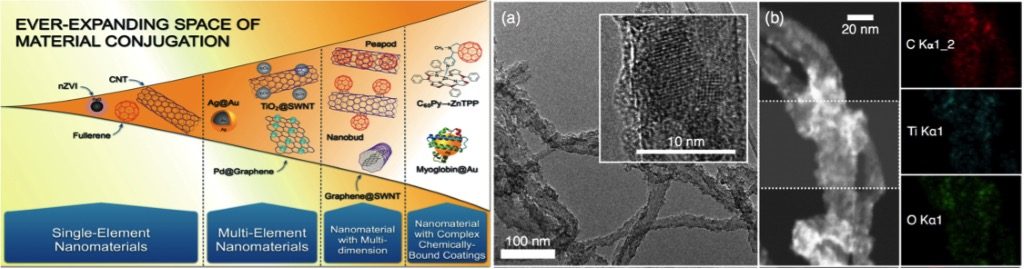
We synthesize and characterize a set of carbonaceous-metal oxide NHs under comparable synthesis conditions, enumerate underlying mechanisms of metal oxide formation on multiwalled carbon nanotubes (MWNT) surfaces, and assess aggregation, deposition, and toxicity of a select NH and its components as a function of the metal oxide loading. A modified sol-gel technique has been developed to grow TiO2, ZnO, Er2O3, and Pr6O11 nanocrystals on MWNT surfaces. Fate, transport, and toxicity of TiO2 hybridized MWNTs for a range of loading (C:Ti molar ratios of 1:0.1, 1:0.05 and 1:0.033) is systematically assessed The nanohybrids are synthesized with a modified sol-gel technique and characterized using transmission electron microscopy, scanning transmission electron microscopy, X-ray photoelectron spectroscopy, and X-ray diffraction. The aggregation kinetics of the nanomaterials is investigated using time resolved dynamic light scattering in presence of mono- and divalent cations and in presence of Suwannee River humic acid (SRHA). The aggregation results suggest that the NHs will strongly aggregate in presence of only NaCl and the MWNTs will show faster aggregation in presence of CaCl2. However, the aggregation behavior of the NHs cannot be captured by that of their component materials and is dependent on the TiO2 loading in the NHs, the hybridization process and the type of cations present. The experimental aggregation results of the NHs show significance deviation from the DLVO theory highlighting the necessity of capturing the complexities associated with the nanohybrid structure and surface chemistry in order to fully understand their aggregation behavior. Presence of SRHA reduces aggregation propensity of the NHs as well as their component materials. Deposition and toxicity of these materials are currently being performed.
Additional Links:
http://www.mdpi.com/2079-4991/5/2/1102/htm
